Why Do Microtubules Continuously Disassemble and Assmeble
Microtubules, the third principal component of the cytoskeleton, are rigid hollow rods approximately 25 nm in diameter. Like actin filaments, microtubules are dynamic structures that undergo continual assembly and disassembly within the cell. They function both to determine cell shape and in a variety of cell movements, including some forms of cell locomotion, the intracellular transport of organelles, and the separation of chromosomes during mitosis.
Structure, Assembly, and Dynamic Instability of Microtubules
In contrast to intermediate filaments, which are composed of a variety of different fibrous proteins, microtubules are composed of a single type of globular protein, called tubulin. Tubulin is a dimer consisting of two closely related 55-kd polypeptides, α-tubulin and β-tubulin. Like actin, both α- and β-tubulin are encoded by small families of related genes. In addition, a third type of tubulin (γ-tubulin) is specifically localized to the centrosome, where it plays a critical role in initiating microtubule assembly (discussed shortly).
Tubulin dimers polymerize to form microtubules, which generally consist of 13 linear protofilaments assembled around a hollow core (Figure 11.37). The protofilaments, which are composed of head-to-tail arrays of tubulin dimers, are arranged in parallel. Consequently, microtubules (like actin filaments) are polar structures with two distinct ends: a fast-growing plus end and a slow-growing minus end. This polarity is an important consideration in determining the direction of movement along microtubules, just as the polarity of actin filaments defines the direction of myosin movement.
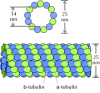
Figure 11.37
Structure of microtubules. Dimers of α- and β-tubulin polymerize to form microtubules, which are composed of 13 protofilaments assembled around a hollow core.
Tubulin dimers can depolymerize as well as polymerize, and microtubules can undergo rapid cycles of assembly and disassembly. Both α- and β-tubulin bind GTP, which functions analogously to the ATP bound to actin to regulate polymerization. In particular, the GTP bound to β-tubulin (though not that bound to α-tubulin) is hydrolyzed to GDP during or shortly after polymerization. This GTP hydrolysis weakens the binding affinity of tubulin for adjacent molecules, thereby favoring depolymerization and resulting in the dynamic behavior of microtubules. Like actin filaments (see Figure 11.4), microtubules undergo treadmilling, a dynamic behavior in which tubulin molecules bound to GDP are continually lost from the minus end and replaced by the addition of tubulin molecules bound to GTP to the plus end of the same microtubule. In microtubules, GTP hydrolysis also results in the behavior known as dynamic instability, in which individual microtubules alternate between cycles of growth and shrinkage (Figure 11.38). Whether a microtubule grows or shrinks is determined by the rate of tubulin addition relative to the rate of GTP hydrolysis. As long as new GTP-bound tubulin molecules are added more rapidly than GTP is hydrolyzed, the microtubule retains a GTP cap at its plus end and microtubule growth continues. However, if the rate of polymerization slows, the GTP bound to tubulin at the plus end of the microtubule will be hydrolyzed to GDP. If this occurs, the GDP-bound tubulin will dissociate, resulting in rapid depolymerization and shrinkage of the microtubule.
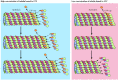
Figure 11.38
Dynamic instability of microtubules. Dynamic instability results from the hydrolysis of GTP bound to β-tubulin during or shortly after polymerization, which reduces its binding affinity for adjacent molecules. Growth of microtubules continues (more...)
Dynamic instability, described by Tim Mitchison and Marc Kirschner in 1984, results in the continual and rapid turnover of most microtubules, which have half-lives of only several minutes within the cell. As discussed later, this rapid turnover of microtubules is particularly critical for the remodeling of the cytoskeleton that occurs during mitosis. Because of the central role of microtubules in mitosis, drugs that affect microtubule assembly are useful not only as experimental tools in cell biology but also in the treatment of cancer. Colchicine and colcemid are examples of commonly used experimental drugs that bind tubulin and inhibit microtubule polymerization, which in turn blocks mitosis. Two related drugs (vincristine and vinblastine) are used in cancer chemotherapy because they selectively inhibit rapidly dividing cells. Another useful drug, taxol, stabilizes microtubules rather than inhibiting their assembly. Such stabilization also blocks cell division, and taxol is used as an anticancer agent as well as an experimental tool.
The Centrosome and Microtubule Organization
The microtubules in most cells extend outward from a microtubule-organizing center, in which the minus ends of microtubules are anchored. In animal cells, the major microtubule-organizing center is the centrosome, which is located adjacent to the nucleus near the center of interphase (nondividing) cells (Figure 11.39). During mitosis, microtubules similarly extend outward from duplicated centrosomes to form the mitotic spindle, which is responsible for the separation and distribution of chromosomes to daughter cells. The centrosome thus plays a key role in determining the intracellular organization of microtubules, although most details of its function remain a mystery.
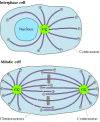
Figure 11.39
Intracellular organization of microtubules. The minus ends of microtubules are anchored in the centrosome. In interphase cells, the centrosome is located near the nucleus and microtubules extend outward to the cell periphery. During mitosis, duplicated (more...)
The centrosome serves as the initiation site for the assembly of microtubules, which grow outward from the centrosome toward the periphery of the cell. This can be clearly visualized in cells that have been treated with colcemid to disassemble their microtubules (Figure 11.40). When the drug is removed, the cells recover and new microtubules can be seen growing outward from the centrosome. Importantly, the initiation of microtubule growth at the centrosome establishes the polarity of microtubules within the cell. In particular, microtubules grow by the addition of tubulin to their plus ends, which extend outward from the centrosome toward the cell periphery.

Figure 11.40
Growth of microtubules from the centrosome. Microtubules in mouse fibroblasts are visualized by immunofluorescence microscopy using an antibody against tubulin. (A) The distribution of microtubules in a normal interphase cell. (B) This cell was treated (more...)
The centrosomes of most animal cells contain a pair of centrioles, oriented perpendicular to each other, surrounded by amorphous pericentriolar material (Figure 11.41). The centrioles are cylindrical structures consisting of nine triplets of microtubules, similar to the basal bodies of cilia and flagella (discussed later in the chapter). Although centrioles are probably the precursors of basal bodies, they appear to be dispensible for the function of the centrosome. Centrioles do not appear to be required for the assembly or organization of microtubules, and they are not found in plant cells, many unicellular eukaryotes, and some animal cells (such as mouse eggs). The microtubules that emanate from the centrosome terminate in the pericentriolar material, not the centrioles, and it is the pericentriolar material that initiates microtubule assembly.

Figure 11.41
Structure of centrosomes. (A) Electron micrograph of a centrosome showing microtubules radiating from the pericentriolar material that surrounds a pair of centrioles. (B) Transverse section of a centriole illustrating its nine triplets of microtubules. (more...)
The key protein in the centrosome that nucleates assembly of microtubules is γ-tubulin, a minor species of tubulin first identified in fungi. Complexes of γ-tubulin form ring structures that contain 10 to 13 γ-tubulin molecules and have diameters similar to those of microtubules. These γ-tubulin rings serve as nucleation sites for the assembly of microtubules and may remain bound to their minus ends.
Reorganization of Microtubules during Mitosis
As noted earlier, microtubules completely reorganize during mitosis, providing a dramatic example of the importance of their dynamic instability. The microtubule array present in interphase cells disassembles and the free tubulin subunits are reassembled to form the mitotic spindle, which is responsible for the separation of daughter chromosomes (Figure 11.42). This restructuring of the microtubule cytoskeleton is directed by duplication of the centrosome to form two separate microtubule-organizing centers at opposite poles of the mitotic spindle.

Figure 11.42
Electron micrograph of the mitotic spindle. The spindle microtubules are attached to condensed chromosomes at metaphase. (From C. L. Rieder and S. S. Bowser, 1985. J. Histochem. Cytochem. 33: 165/Biological Photo Service.)
The centrioles and other components of the centrosome are duplicated in interphase cells, but they remain together on one side of the nucleus until the beginning of mitosis (Figure 11.43). The two centrosomes then separate and move to opposite sides of the nucleus, forming the two poles of the mitotic spindle. As the cell enters mitosis, the dynamics of microtubule assembly and disassembly also change dramatically. First, the rate of microtubule disassembly increases about tenfold, resulting in overall depolymerization and shrinkage of microtubules. At the same time, the number of microtubules emanating from the centrosome increases by five- to tenfold. In combination, these changes result in disassembly of the interphase microtubules and the outgrowth of large numbers of short microtubules from the centrosomes.

Figure 11.43
Formation of the mitotic spindle. The centrioles and centrosomes duplicate during interphase. During prophase of mitosis, the duplicated centrosomes separate and move to opposite sides of the nucleus. The nuclear envelope then disassembles, and microtubules (more...)
As first proposed by Marc Kirschner and Tim Mitchison in 1986, formation of the mitotic spindle involves the selective stabilization of some of the microtubules radiating from the centrosomes. These microtubules are of three types, two of which make up the mitotic spindle. Kinetochore microtubules attach to the condensed chromosomes of mitotic cells at their centromeres, which are associated with specific proteins to form the kinetochore (see Figure 4.16). Attachment to the kinetochore stabilizes these microtubules, which, as discussed below, play a critical role in separation of the mitotic chromosomes. The second type of microtubules found in the mitotic spindle (polar microtubules) are not attached to chromosomes. Instead, the polar microtubules emanating from the two centrosomes are stabilized by overlapping with each other in the center of the cell. Astral microtubules extend outward from the centrosomes to the cell periphery and have freely exposed plus ends. As discussed later, both the polar and astral microtubules also contribute to chromosome movement by pushing the spindle poles apart.
As mitosis proceeds, the condensed chromosomes first align on the metaphase plate and then separate, with the two chromatids of each chromosome being pulled to opposite poles of the spindle. Chromosome movement is mediated by motor proteins associated with the spindle microtubules, as will be discussed shortly. In the final stage of mitosis, nuclear envelopes re-form, the chromosomes decondense, and cytokinesis takes place. Each daughter cell then contains one centrosome, which nucleates the formation of a new network of interphase microtubules.
Stabilization of Microtubules and Cell Polarity
Because of their inherent dynamic instability, most microtubules are frequently disassembled within the cell. This dynamic behavior can, however, be modified by the interactions of microtubules with other proteins. Some cellular proteins act to disassemble microtubules, either by severing microtubules or by increasing the rate of tubulin depolymerization from microtubule ends. Other proteins (called microtubule-associated proteins or MAPs) bind to microtubules and increase their stability. Such interactions allow the cell to stabilize microtubules in particular locations and provide an important mechanism for determining cell shape and polarity.
A large number of MAPs have been identified, and they vary depending on the type of cell. The best-characterized are MAP-1, MAP-2, and tau, isolated from neuronal cells, and MAP-4, which is present in all non-neuronal vertebrate cell types. The tau protein has been extensively studied because it is the main component of the characteristic lesions found in the brains of Alzheimer patients. The activity of MAPs is regulated by phosphorylation, allowing the cell to control microtubule stability.
A good example of the role of stable microtubules in determining cell polarity is provided by nerve cells, which consist of two distinct types of processes (axons and dendrites) extending from a cell body (Figure 11.44). Both axons and dendrites are supported by stable microtubules, together with the neurofilaments discussed in the preceding section of this chapter. However, the microtubules in axons and dendrites are organized differently and associated with distinct MAPs. In axons, the microtubules are all oriented with their plus ends away from the cell body, similar to the general orientation of microtubules in other cell types. The minus ends of most of the microtubules in axons, however, are not anchored in the centrosome; instead, both the plus and minus ends of these microtubules terminate in the cytoplasm of the axon. In dendrites, the microtubules are oriented in both directions; some plus ends point toward the cell body and some point toward the cell periphery. These distinct microtubule arrangements are paralleled by differences in MAPs: Axons contain tau proteins, but no MAP-2, whereas dendrites contain MAP-2, but no tau proteins, and it appears that these differences in MAP-2 and tau distribution are responsible for the distinct organization of stable microtubules in axons and dendrites.
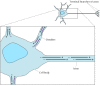
Figure 11.44
Organization of microtubules in nerve cells. Two distinct types of processes extend from the cell body of nerve cells (neurons). Dendrites are short processes that receive stimuli from other nerve cells. The single long axon then carries impulses from (more...)


callaghancamse1949.blogspot.com
Source: https://www.ncbi.nlm.nih.gov/books/NBK9932/
0 Response to "Why Do Microtubules Continuously Disassemble and Assmeble"
Post a Comment